
SOLVED: A 40.0 ml sample of 0.01 M ethanoic acid (CH3COOH) is titrated with 0.02 M sodium hydroxide, NaOH. Calculate the pH after adding 30.0 ml of NaOH solution. *Given Ka of
When a solution of 0.01 M CH3COOH is titrated with a solution of 0.01 M NaOH. Calculate the pH at the equivalence point. - Sarthaks eConnect | Largest Online Education Community
Calculate the pH of 0.1M CH3COOH solution. Dissociation constant of acetic acid is 1.8 x 10^-5 - Sarthaks eConnect | Largest Online Education Community
Calculate the pH of a 0.01 M solution of acetic acid. Ka for CH3COOH is 1.8 x 10^-5 at 25°C. - Sarthaks eConnect | Largest Online Education Community
pH of 0.1 M CH3COOH solution is 3 at 25 degree celcius . if limiting molar conductivity of CH3COO and H+ are 40 and 350 S cm2 mol . the molar conductance at 25 degree for 0.1M CH3COOH
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://haygot.s3.amazonaws.com/questions/1938773_1231298_ans_7f19b0e0d221405fbf38b0df680608aa.jpg)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]
![The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube](https://i.ytimg.com/vi/6n7yj8M7Dp4/maxresdefault.jpg)
The `[H^(+)]` of a resulting solution that is 0.01 M acetic acid `(K_(a)=1.8xx10^(-5))` and 0.01... - YouTube
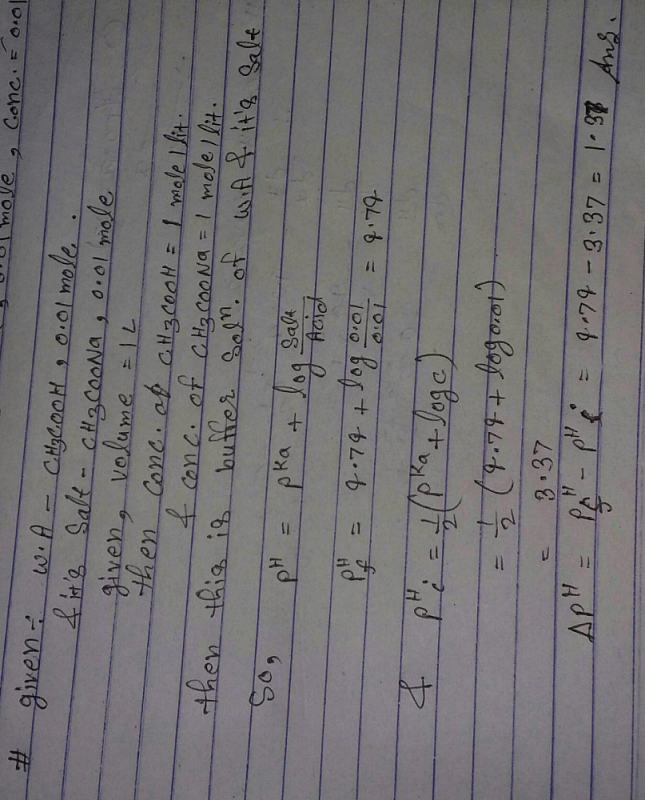
Find the change in pH ,when 0.01 mole CH3COONA is added to one liter of 0.01M CH3COOH solution (pKa =4.74) ??? ; ANS:1.37? | EduRev Class 11 Question

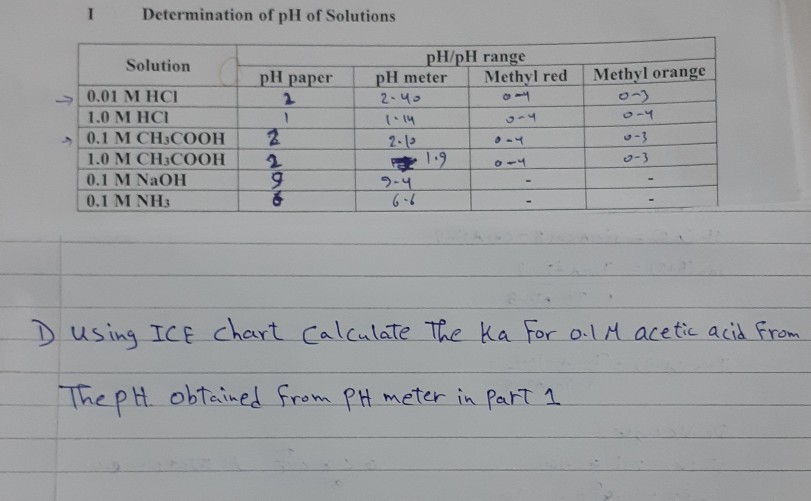

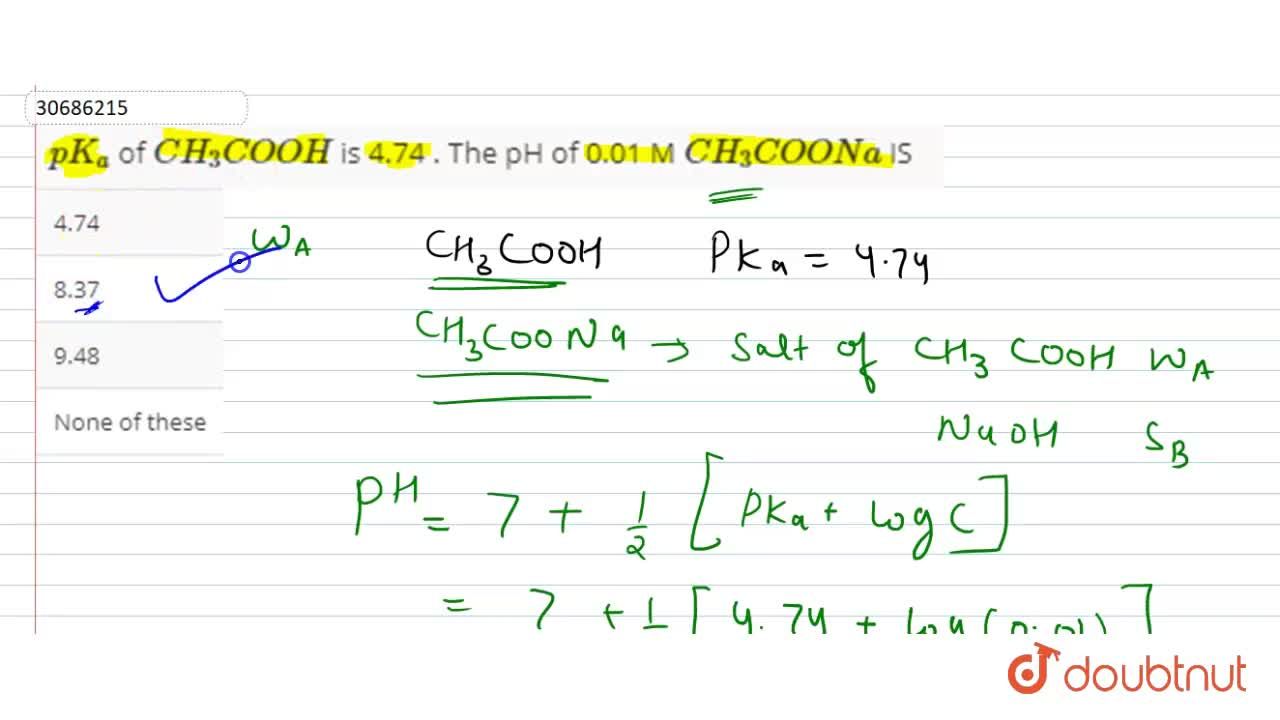
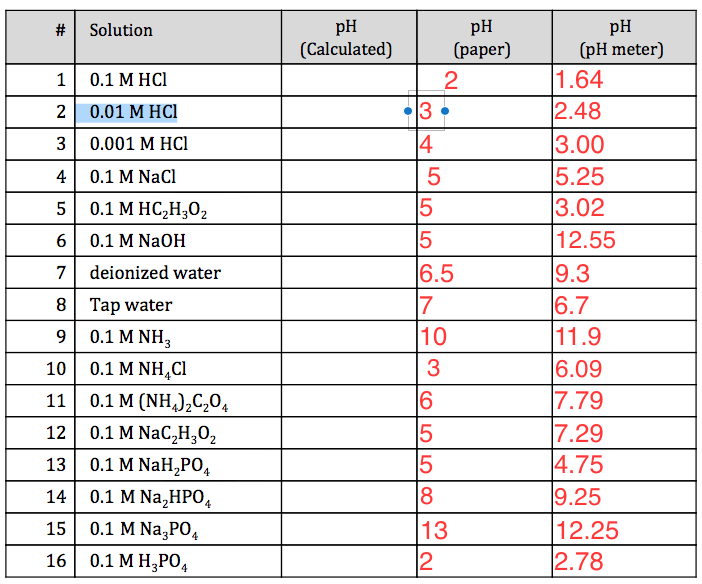
![The pH of 0.01 M solution of acetic acid is 5.0. What are the values of [H^+] and Ka respectively? The pH of 0.01 M solution of acetic acid is 5.0. What are the values of [H^+] and Ka respectively?](https://haygot.s3.amazonaws.com/questions/1445613_1156485_ans_e20f838d2ffe4d0eb21c5b51d09eb783.jpg)







