10 ml of a solution having PH =4 is added with 990 ml of 0.1M NaCl solution. PH of resulting solution - Brainly.in
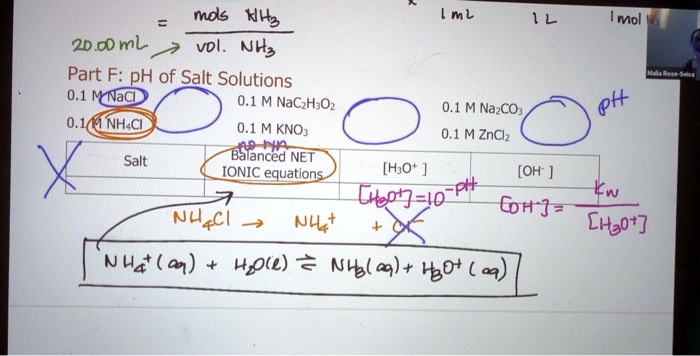
SOLVED: mds Nh? ml Mol 2D.@ml Vdl Nhs Part F: pH of Salt Solutions Nacl 0.1 M NaCzH;Oz 0.1 M NazCOz Ph NHACI 0.1 M KNO 0.1 M ZnClz Salt Balanced NET
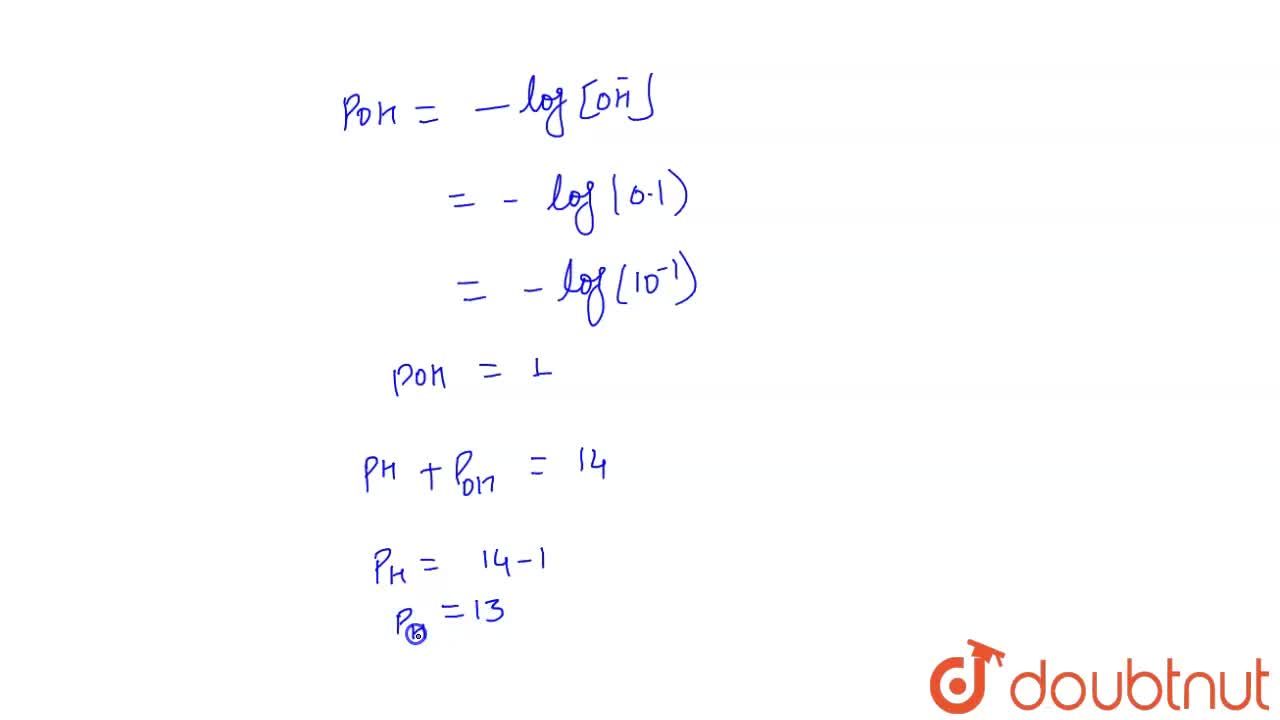
Calculate the pH of 0.5 of 1.0 M NaCl solution after electrolysis when a current of 5.0 ampere is passed for 965 seconds.

Evolution of the open circuit potential for AMlite in 0.1 M NaCl at pH... | Download Scientific Diagram

Dependence of M-L-Gln hydrodynamic radius, Rh, on: (a) pH (in 0.1 M... | Download Scientific Diagram

A weak acid HX has the dissociation constant value of 1 × 10^-5 M . It forms a salt NaX on reaction with NaOH . The percentage degree of hydrolysis of 0.1 M solution of NaX is: